Chemistry
You are logged in as xxxx. Logout
Question
 Your answer agrees with the answer suggested by the author, and is the most popular answer
Your answer agrees with the answer suggested by the author, and is the most popular answer
This question has been answered by 122 people and has an average rating of 4.17 (based on 69 ratings)
| Quality rating | Total | Difficulty ratings (easy, medium, hard) |
| Excellent | 27 | xxxxxxxxxxxxxxxxxxxxxxxxxxx |
| Very good | 32 | xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx |
| Good | 8 | xxxxxxxx |
| Fair | 0 | |
| Poor | 1 | x |
| Very poor | 1 | x |
Which one of the following ions is most stable?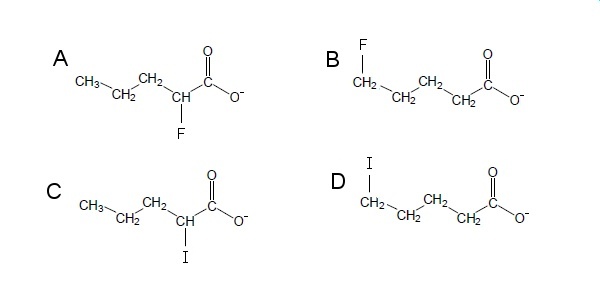
Alternatives
| Option | Alternative | First answers |
Confirmed answers |
|---|---|---|---|
| A |
A |
58 (47.54%) |
39 (97.50%) |
| B |
B |
11 (9.02%) |
0 (0.00%) |
| C |
C |
43 (35.25%) |
1 (2.50%) |
| D |
D |
10 (8.20%) |
0 (0.00%) |
Explanation
The following explanation has been provided relating to this question:
Topics
The following topics have been indicated as being relevant to this question:
Comments
There are 26 comments for this question (26 top-level comments and 0 replies)  You may choose to agree or disagree
with any of the comments previously written about this question (except
comments you have written). Select either the star (agree) or the
cross (disagree) to the right of the comments that you feel most
strongly about. The order in which comments about this question will be
displayed will be determined by the level of agreement
You may choose to agree or disagree
with any of the comments previously written about this question (except
comments you have written). Select either the star (agree) or the
cross (disagree) to the right of the comments that you feel most
strongly about. The order in which comments about this question will be
displayed will be determined by the level of agreement
 You may choose to agree or disagree
with any of the comments previously written about this question (except
comments you have written). Select either the star (agree) or the
cross (disagree) to the right of the comments that you feel most
strongly about. The order in which comments about this question will be
displayed will be determined by the level of agreement
You may choose to agree or disagree
with any of the comments previously written about this question (except
comments you have written). Select either the star (agree) or the
cross (disagree) to the right of the comments that you feel most
strongly about. The order in which comments about this question will be
displayed will be determined by the level of agreementFollow
 |
If you would like to follow this author, click the "Follow" button. This will give you access to all of their existing and new questions in the "Followed questions" section of the Main Menu. |
There are two contributing factors to this question, one is distance and another is the electronegativity.
First, when the halogen group is further from the site of dense charge, the induction effect becomes weaker, therefore there is a lesser contribution to the stability of the ion.
Second, as the electronegativity of an atom increases, the dipole created is stronger. In acid conditions, pKa decreases slightly due to this strong electronegativity, making an even more acidic compound. The more acidic the compound is, the more stable its conjugate base will be.
Request help
Improve explanation